| Sr. No. | Product | Manufacturer | Collaborator |
| 1 | Covishield | Serum Institute of India, Pune | Astra Zeneca |
| 2 | Covaxin (inactivated Virus) | Bharat Biotech International Ltd., Hyderabad | Indian Council of medical Research, India |
| 3 | ZyCoV-D (DNA Vaccine) | Cadila Healthcare Ltd., Ahmedabad (Zyduc Cadila) | Dept. of Biotechnology, India |
| 4 | Sputnik-V (Human Adenovirus Vaccine) | Trialed & Manufactured in India by Dr. Reddy’s Lab | Gamaleya National Center, Russia |
| 5 | NXV-CoV2373 | Serum Institute of India, Pune | Novavax |
| 6 | Recombinant Protein Antigen based Vaccine | Biological E Ltd., Hyderabad | MIT, USA |
| 7 | HGCO 19 (mRNA-based vaccine) | Genova | HDT, USA |
| 8 | Inactived Rabies vector platform | Bharat Biotech International Ltd., Hyderabad | Indian Council of Medical Research, India |
| 9 | Vesiculo Vax Platform | Aurobindo Pharma Ltd., Hyderabad | Aurovaccine, USA |
Current Govt. Guidelines on Covid Administration

The priority group of above 50 years may be further subdivided into those above 60 years of age and those between 50 to 60 years of age for purposes of phasing of roll out based on pandemic situation and vaccine availability.
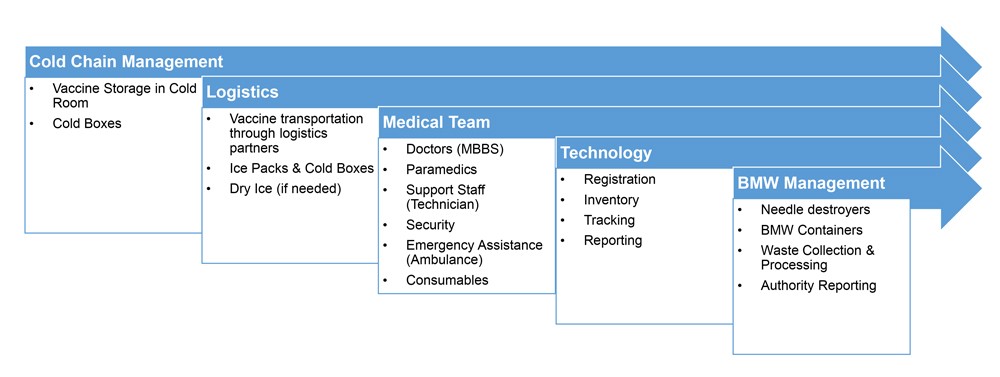
COVID Vaccine Administration Process

Operating Model with Clients & Partners